Viral Diseases of Aquarium Fishes
Author: Jay Hemdal
A longtime aquarist and current Curator of Fishes at the Toledo Zoo describes the illnesses that may afflict your fish beyond the commonly seen ich.
Handling Viral Diseases
As home aquarists gain experience they learn how to deal with a variety of common diseases that may afflict their fish. Common protozoan diseases, such as ich, give fairly straightforward symptoms, and there are many products available on the market to help aquarists deal with those outbreaks. Other diseases, such as Uronema, are more difficult to diagnose because their symptoms mimic other problems (Uronema is often misidentified as a bacterial infection). Viral diseases are the most difficult for home aquarists to deal with in their fishes. Symptoms can match those of a number of other problems, and even if the aquarist can properly identify a virus as the root cause of a problem, there is simply no cure available.
There are three important aspects of handling viral diseases in aquarium fish:
1) Making certain that one does not misidentify the problem and begin a possibly harmful incorrect treatment.
2) Once the problem is properly identified, isolating the infected fish so the viral disease does not spread to other fishes.
3) Offering the infected fish supportive care so it stands a better chance of recovering from the viral infection on its own.
What Are Viruses?
Viruses are small infectious bodies that replicate themselves inside the cells of living things. Different viruses infect literally all types of organisms: animals, plants, and bacteria. They possess some of the characteristics of other living organisms and are affected by natural selection, but they cannot replicate themselves without first invading a host cell. Virus particles consist of particular genetic material (DNA or RNA), covered by a protective layer of protein and, in some cases, an envelope of fat molecules that surrounds the protein layer. A typical virus is a hundred times smaller than the average bacterial cell and is too small to be seen through a regular microscope.
Viruses spread in a variety of ways: through direct contact, through air or water, by their host animals being eaten by another, or even by being shed in feces. Each type of virus has a range of host cells that it is able to infect. Some viruses can infect many different species, while others can infect only a few closely related types of fishes.
Viral Infections in Fishes
Over 125 different viruses have been documented in fishes (Noga 2010), but most of them have been in aquacultured food fishes, as that is where the most resources are focused. Symptoms of piscine viral infections overlap those resulting from some other diseases, so positive identification is difficult with aquarium fishes. Although vaccines are available to protect some species of food fishes from some viruses, it needs to be understood that no medications are available to treat active viral diseases in any fish.
Angelfish Virus
Twenty-five to 30 years ago, normally hardy freshwater angelfish varieties began dying like flies in pet stores and their customers’ aquariums alike. The causative agent was determined to be a virus, simply by lack of any other definitive diagnosis. It may be that there was more than one infectious agent at work or that fish compromised by one disease became susceptible to others. One study did isolate a virus from affected angelfish. This disease, viral hematopoietic necrosis (VHN), was thought to be caused by an iridovirus (Schuh & Shirley 1990). This disease causes high mortality in angelfish through necrosis of their internal organs.
Banggai Cardinalfish Iridovirus (BCIR)
In the early 1990s, when the Banggai cardinalfish (Pterapogon kauderni) was “rediscovered” and began entering the tropical fish trade, aquarists noted how hardy the species was and that captive individuals were relatively easy to reproduce. A decade later the price for wild-caught Banggai cardinalfish had decreased dramatically, but the animals were deemed much more delicate.
Poor handling, collection with cyanide (unlikely), and bacterial disease were all suggested as reasons for this change. A researcher demonstrated the presence of an iridovirus associated with episodes of mass mortality in newly imported cardinalfish (Weber, et-al 2009). While there is no known cure for this disease, captive-raised fish that have never been exposed to wild stock, or fish that have developed immunity by surviving an infection, would be the best choices for home aquariums.
Carp Pox
Affecting mainly the carp (Cyprinus carpio), this viral disease is important only to aquarists who have ponds, as it infects only koi and other ornamental carp. This herpes1 virus (Herpesvirus cyprini) causes raised lesions (papillomas) that, while unsightly, are rarely fatal to the infected fish. Occasionally there is a problem with secondary bacterial infections of the open sores. Eventually the disease goes into remission as the fish develops immunity to the virus, but some fish develop permanent scarring. In many ways this disease is similar to chickenpox in humans.
Koi Herpes Virus (KHV)
Another viral disease that is of concern to people who keep koi in ponds, this disease seems to mainly affect the koi’s gills and is most commonly seen when pond water temperatures are between 72° and 78°F (22° and 25.5°C). Symptoms include white-mottled gills and sunken eyes. The gill damage impairs the fish’s ability to extract oxygen from the water, so fish gasping at the surface may be noticed.
Dwarf Gourami Iridovirus (DGIR)
About 10 years ago, aquarium dealers and hobbyists noticed a greatly increased mortality rate in dwarf gouramis (Trichogaster lalius) raised on fish farms in Southeast Asia and imported for the pet trade. The apparent cause is an iridovirus that causes necrosis of the spleen and kidney in infected fish. Symptoms are varied and include pale coloration, loss of appetite, lesions on the body, ascites (abdominal swelling), and a very high mortality rate. While other species of gourami do not seem to be as strongly affected, a food fish, the Murray cod (Maccullochella peelii peelii), is susceptible to the same virus (Go, et al. 2006). As with other viral diseases, there is little home aquarists can do about it other than try to avoid acquiring infected animals. As this disease seems to have a swift onset, avoiding the purchase of any recently imported dwarf gouramis is the best course of action.
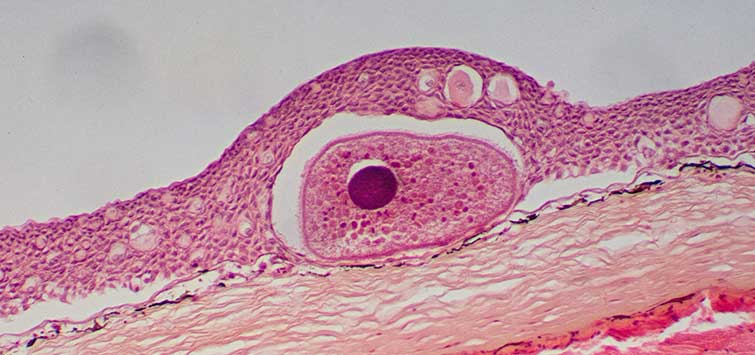
Lymphocystis/Cauliflower Disease
This is a common viral disease of marine, brackish, and some freshwater fishes. It is a chronic (long-lasting) but self-limiting (usually going away on its own) syndrome caused by an iridovirus. The virus causes hypertrophy, or enlargement, of the epithelial cells of a fish’s skin and fins. Initial symptoms consist of off-white to gray nodules on the fish that spread and grow larger over a timeframe of 10 to 30 days.
Lymphocystis can be confused initially with protozoan infections, such as those caused by Cryptocaryon or Ichthyophthirius (marine or freshwater ich, respectively), or even a fungal infection caused by Saprolegnia. Eventually the fact that the lesions do not progress rapidly to an acute phase shows that they are not caused by these more virulent diseases. It is most often seen in newly imported fishes, so capture and transport stress is commonly cited as the instigating factor for this disease. While this may be true, it may also be that the cause is actually exposure to other infected fishes in the aquarium systems of the exporter, importer, and retail suppliers. In any event, it is extremely rare for a fish held in captivity for more than four to six months to subsequently develop this disease.
Lymphocystis infections can become acute, covering large areas of a fish’s body and even impeding feeding if the enlarged cell growth involves the mouth. In rare instances the virus can also cause hypertrophy of internal cells, especially in marine fishes (Wolf 1988).
A variety of cures have been promoted for this disease over the years. Some public aquarists have reported that a reduction in the animal’s environmental stress level will help. Others have reported that a treatment with a mixture of malachite green and formalin helps limit the spread of the lesions. By far the most commonly promoted treatment consists of surgically removing the hypertrophied skin cells, usually followed with a topical antibiotic to, hopefully, prevent secondary bacterial infection.
The problem with these purported treatments is that, since the disease is usually self-limiting, remission of the lymphocystis almost always occurs in spite of any treatment offered. Secondly, surgical excision of the lesions releases viral particles into the aquarium, possibly spreading the disease. Finally, any time a fish is handled there is a risk to its health due to injury or infection. The general advice is to never try to treat a lymphocystis infection—just let it run its course.
The distribution of this disease in aquarium fishes may have changed over time. In the 1970s it was a very common problem in some species, and even beginning aquarists could recognize it. More recently it seems to have become less commonly seen, and when it does appear, aquarists are often unable to diagnose it properly because they have not had prior experience with it.
To put this disease into perspective, out of 407 fishes necropsied at a public aquarium over 10 years, lymphocystis was identified as the cause of death in only 1 percent of the cases, as compared to protozoan disease that caused 26 percent of the fish deaths (Hemdal 2006).
Although lymphocystis infections are fairly distinctive, there are some other fish diseases that can be confused with it. Epistylis is an opportunistic stalked protozoan that can form white colonies on a fish’s body, especially at the site of a previous injury. It can take time to develop and is rarely fatal, but unlike with lymphocystis, the lesions rarely go away without treatment. Lesions formed by Epistylis are rounder and have less distinctive edges than lymphocystis does. Microscopically, Epistylis can be readily identified as a protozoan.
In freshwater fishes, early Saprolegnia fungal infections are sometimes misidentified as lymphocystis, but fungal infections have less distinctive edges and can easily be identified by examining a scraping under a low-powered microscope.
For unknown reasons, some marine fishes may begin to produce excess mucus on the edges of their fins. These white lesions may also resemble lymphocystis but will appear smoother, and under a microscope a scraping will show only round mucus cells.
Similar lesions are caused by another virus, Walleye Dermal Sarcoma (WDS), which causes large round pinkish growths on the skin of walleyes in the Great Lakes region. The condition is rarely fatal but may make the fish unappealing to anglers who mistakenly think it is caused by polluted water or exposure to radiation.
HLLE and Virus Disease
One research project has associated the development of head and lateral line erosion (HLLE) with the presence of a reovirus in marine angelfish (Varner & Lewis 1991). However, this study did not clearly show that the virus caused the HLLE, and that problem may just have been coincidental to the angelfish’s having been infected with the virus. Transmission of HLLE by an infectious agent was not duplicated in a subsequent study (Hemdal & Odum 2011) that showed that the use of activated lignite carbon was the causative agent in the development of HLLE in a group of surgeonfish.
Additionally, it has been shown anecdotally that HLLE lesions can often be alleviated by moving the affected fish to a new environment. One would expect that if a virus were associated with this syndrome, moving the fish would transport the virus along with it, but this has not been the case. It could possibly be that the HLLE lesions actually weaken the fish’s immune system, making it susceptible to a subsequent viral infection.
Control of Viral Diseases
If you suspect that a fish under your care is infected with a viral disease, the best course of action is to limit the potential for its spread to other fishes. Isolating all possible means of transmission is the first thing to do. Nets or any other aquarium equipment exposed to the water in a contaminated aquarium must be sterilized before re-use in another aquarium.
Aerosolization is another means by which viral particles (virions) may be transmitted from one aquarium to another. These virions are so small that an air bubble bursting at the surface of an aquarium can propel them up into the air, and they can then settle out in other nearby aquaria.
For the infected fish itself, since there is no cure for viral diseases, all you can hope for is that it will recover on its own. You can help this process along by giving the fish the best possible care during the time it shows symptoms of the infection.
Some viral diseases are temperature dependent and do not grow well at certain temperatures. However, this is difficult to use as a control for viral diseases in captive fishes, as many tropicals have a narrower survivable temperature range than does the virus.
Sodium hypochlorite is a commonly used net-dip chemical. A disinfecting solution can be prepared by mixing one part regular household bleach (5.25% sodium hypochlorite solution, with no fragrances or dyes) to nine parts tap water. Always wear eye protection and gloves when handling bleach. Store the solution in a closed container away from pets and children. After use in an aquarium, nets and tank tools are then dipped in this solution for ten minutes and hung up to dry. Items thus treated should always be rinsed well with tap water before using them in another aquarium. New net-dip solution needs to be prepared every two to four weeks, depending on usage (organic material adhering to the nets tends to inactivate the disinfecting properties of the bleach).
Recovery and Quarantine
Following the hoped-for recovery of the fish infected with a viral disease, the next question is, will it be safe to now move the fish to an aquarium with uninfected fishes? Unfortunately, the answer to this is pretty much unknown. While that individual has recovered and has built up immunity to that particular virus, there is little information available to say whether it is non-infective to fishes that have not been exposed to the virus.
Certainly, keeping the fish in isolation for as long as possible after its remission would be prudent. There will always be some degree of doubt about this, so in order to minimize risk it is best to never place that fish in an aquarium with other, possibly irreplaceable, specimens.
Fisheries biologists working with game fish production in fish farms will often opt to euthanize these individuals and dispose of them in a landfill to ensure that viral particles do not spread to other fishes on the farm. However, home aquarists will need to make a judgment call, weighing the risks versus the benefits of keeping their fish post-infection.
Works Cited
Go, J., M. Lancaster, K. Deece, O. Dhungyel, and R. Whittington. “The Molecular Epidemiology of Iridovirus in Murray Cod (Maccullochella peelii peelii) and Dwarf Gourami (Colisa lalia) from Distant Biogeographical Regions Suggests a Link between Trade in Ornamental Fish and Emerging Iridoviral Diseases.” Molecular and Cellular Probes 20, no. 3–4 (June-August 2006): 212–22.
Hemdal, J. F. Advanced Marine Aquarium Techniques. Neptune City: TFH Publications, 2006.
Hemdal, J. F., and R. A. Odum. “The Role of Activated Lignite Carbon in the Development of Head and Lateral Line Erosion in the Ocean Surgeon.” North American Journal of Aquaculture 73, no. 4 (2011): 489–92.
Noga, E. J. Fish Disease: Diagnosis and Treatment. Hoboken: John Wiley & Sons, Inc., 2010.
Schuh, J. C. L., and I. G. Shirley. “Viral Hematopoietic Necrosis in an Angelfish (Pterophyllum scalare).” Journal of Zoo and Wildlife Medicine 21, no. 1 (1990): 95–98.
Varner, P. W., and D. H. Lewis. “Characterization of a Virus Associated with Head and Lateral Line Erosion Syndrome in Marine Angelfish.” Journal of Aquatic Animal Health 3, no. 3 (1991): 198–205.
Weber, E. S., III, T. B. Waltzek, D. A. Young, E. L. Twitchell, A. E. Gates, A. Vagelli, G. R. Risatti, R. P. Hedrick, and S. Frasca. “Systemic Iridovirus Infection in the Banggai Cardinalfish (Pterapogon kauderni Koumans 1933).” J Vet Diagn Invest 21, no. 3 (May 2009): 306–20.
Wolf, Ken. Fish Viruses and Fish Viral Diseases. Ithaca: Cornell University Press, 1988. D
See the full article on TFH Digital http://www.tfhdigital.com/tfh/august_2014#pg49

.png?h=595&iar=0&w=2781&hash=5FD5E69473BCC22199FBFA2FB71B6033)