Mycobacteriosis, the Stealth Disease
Author: Diana Walstad
A look at "MB," a common, highly contagious, and incurable bacterial fish disease, and some steps that can be taken to prevent its outbreak.
Table 1: Characteristics of NTM (nontuberculous mycobacteria)
· Gram positive, acid-fast staining, aerobic, non-motile rods.
· Cell wall is lipid-rich and contains waxy mycolic acids that protect NTM from chemicals and disinfectants.
· Extremely slow growth rate puts NTM at a competitive disadvantage in nutrient-rich environments where other bacteria can easily outgrow them.
· Extreme tenacity and ability to grow under nutrient-deprived, starvation conditions.
· Non-sporulating, but NTM can survive for years within the cysts of protozoa.
· Readily form biofilms. Biofilms look like slime and debris to us, but they are actually complex microbial ecosystems of bacteria and protozoa. They are formed wherever water interfaces with air (e.g., “scum” on water surface of aquariums and ponds) or a solid surface (e.g., aquarium filters and inner walls of distribution pipes in drinking water systems).
· Pathogenic NTM can survive and multiply in phagocytic cells (e.g., amoeba and macrophages) that kill ordinary bacteria
Table 2: Concentration of Bleach (ppm) Required to Eradicate NTM (Bardouniotis 2003)
Species Condition of Bacteria Treatment Time: 30 min Treatment Time: 2 hours
M. fortuitum Suspended 53 ppm 53 ppm
Biofilm 2,000 500
M. marinum Suspended <13 <13
Biofilm 26 26
My Experience with Rainbowfish
I have kept and bred rainbowfish for 20 years, and except for a single bout with ich (the common white spot disease), the fish have been remarkably problem-free. For example, two of my male rainbowfish are at least 15 years old and as robust today as their younger tankmates. However, in 2004, after adding new fish to my aquariums, the new fish died or developed strange body sores. Antibiotics did not help. When symptoms appeared on tankmates—fish I had raised from eggs and knew were healthy—I suspected an infectious disease.
A fish veterinarian examined two fish, the only symptoms being some tissue erosion of one fish’s jaw. However, a histological examination showed that the internal organs of both fish were riddled with granulomas containing acid-fast bacteria. The verdict was that my fish had “MB” or mycobacteriosis, a common bacterial disease in fish that is highly contagious and incurable. How distressing! My fish were all going to die from MB. Moreover, the infection could be transmitted to me, resulting in painful, slow-healing sores (Pro 2003).
Dealing with MB
The recommended course was to tear down the tanks, disinfect everything, and start over. However, my three established tanks contained fish and plants that I had kept for many years. So I added a UV sterilizer (equipped with an 8-watt UV lamp) to each of the three tanks (45, 50, and 55 gallons). Water from the biofilter flowed through the UV sterilizer before returning to the tank. The UV sterilizers were designed so that the water flowed in a spiral path around the UV lamp. This design, coupled with a gentle flow rate, maximized the water’s exposure to the sterilizing UV light. The results were amazing! The dying and the appearance of new symptoms decreased dramatically. Some of the fish with symptoms (swollen bodies or sores) actually healed and recovered. The UV’s killing of water-borne bacteria and parasites had perhaps given my fish time to fight off disease, either MB or secondary infections (MB weakens the immune system, making fish vulnerable to other diseases).
I decided to see how contaminated the tanks were by purchasing eight new rainbowfish from a trusted source. Except for one death, the fish did fine. After eight months a fish veterinarian examined three of the new fish, one from each tank. A histological exam showed no granulomas. The disease had not infected the new fish. The fact that I permanently removed the UV sterilizers a few months beforehand make these results even more impressive.
MB Basics
MB (mycobacteriosis), the chronic disease of fish and reptiles, was first documented in diseased carp in 1897. Over the years the disease has not abated. It probably causes many more problems than hobbyists realize. For example, in a random survey of 312 aquarium fish at various fish distribution centers, Praero (2004) found MB in 12 percent of the fish. In a survey of 70 dead aquarium fish, Lescenko (2003) found that 41 percent had MB. Many experts consider MB to be the most common chronic disease in tropical aquarium fish (Astrofsky 2000).
Effects
Because MB has no clear symptoms and can only be confirmed by a histological examination, hobbyists understandably underestimate its prevalence. If a newly purchased fish stops eating and dies after a few weeks, most hobbyists do not suspect MB (much less know what it is). Additionally, chronic MB weakens the fish’s immune system such that infected fish are highly vulnerable to other diseases. For example, when Talaat (1998) experimentally infected goldfish with pathogenic NTM, the chronically infected fish developed the parasitic disease ich (control fish without MB developed no ich). I wonder how many hobbyists have attributed their fish’s disease (or death) to parasites and other pathogens, when the underlying problem was chronic MB.
Characteristics
The bacterial genus Mycobacterium has some very interesting and unique characteristics (Table 1). The genus is roughly subdivided into two groups: 1.) obligate pathogens like M. tuberculosis and M. leprae, which cause tuberculosis and leprosy, respectively, and do not live outside their human hosts; and 2.) NTM (nontuberculous mycobacteria), which live everywhere in the natural environment feeding on organic debris. NTM can, under the right circumstances, cause disease, but that is not the norm. M. marinum, M. fortuitum, and M. chelonae are commonly cited as the species responsible for MB. However, investigators using new genetic methods now frequently isolate other NTM species from diseased fish (Herbst 2001; Kent 2004; Poort 2006; Rhodes 2004; Sakai 2005; Whipps 2003). I believe that almost any NTM species can produce MB in fish, provided it is present in sufficient numbers.
MB involves the “hijacking” of the macrophages, a major player of the animal’s immune system. These large white blood cells ordinarily engulf and digest invading bacteria via phagocytosis. However, macrophages cannot kill pathogenic NTM. Instead, the engulfed NTM survive and multiply inside the macrophages. Infected macrophages travel throughout the animal’s body, starting infections in new tissues.
What Happens to Infected Fish?
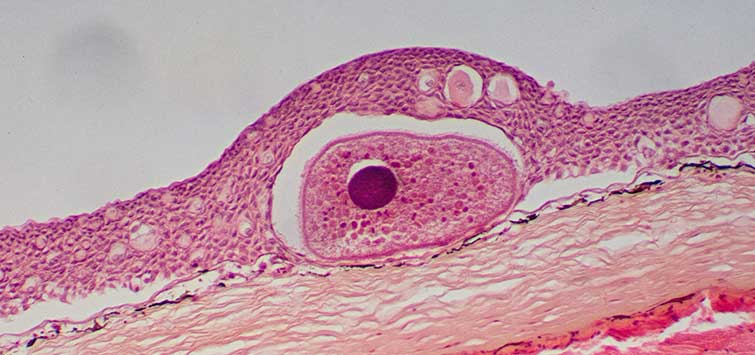
Fish may die within two to eight weeks from a major attack by a virulent NTM, such as M. marinum. Typically, however, NTM infections cause chronic disease. After the fish’s macrophages capture the bacteria, granulomas (round nodules of 0.05 to 4 mm diameter within the fish’s tissues) develop after a few weeks. Inside the granulomas, bacteria, macrophages, and other cells wage a lengthy war of attrition. If the animal’s immune system diminishes, the bacteria may break out of the granulomas, multiply unchecked, and kill the fish. On the other hand, mildly infected fish may be able to eventually rid themselves of disease. Thus, Gauthier (2003) found healed and healing granulomas in mildly infected fish.
NTM: Not Necessarily Pathogens
The same species that cause fish MB are also found throughout nature in soils, lakes, and oceans. For the most part they are saprophytic, that is, feeding on decaying organic matter. They are particularly enriched in environments such as highly acidic swamps (Kirschner 1992), that inhibit the growth of ordinary bacteria.
Healthy aquariums have a natural NTM flora. Beran et al. (2006) screened six well-established, apparently normal aquariums for NTM. The investigators isolated numerous NTM species (e.g., M. fortuitum, M. chelonae, etc.) from the environment (snails, filters, surface water biofilms, plants, fish, etc.). None of the 19 fish autopsied had the granulomas characteristic of MB. The investigators did find NTM in the fish’s tissues, though.
It is surprising that apparently undiseased fish contained NTM, including two NTM species known to be fish pathogens. However, I would argue that the mere presence of NTM does not mean incipient MB. The NTM that Beran et al. (2006) found may not have been that virulent (strains of NTM species can differ greatly in virulence [van der Sar 2004]). Most likely, though, the number of NTM was insufficient to cause disease. Indeed, studies like this only list species found; they do not tell us the actual numbers of NTM. The six normal tanks could conceivably contain the same NTM species as diseased tanks, but the NTM may be present in much smaller numbers.
Disinfection and Cleanliness Enrich for NTM
Ironically, conscientious fish breeders greatly increase the chances of MB by routinely disinfecting tanks. NTM are much more resistant to most chemicals (antibiotics, bleaches, detergents, etc.) than other bacteria. For example, NTM are equally sensitive to ozone and UV, but they are about 10 to100 times more resistant to chlorine and chloramine than the ordinary bacterium Escherichia coli (LeChevallier 2004).
Lab Techniques
The laboratory techniques required to isolate and culture NTM provide a perfect example of how disinfectants and antibiotics kill ordinary bacteria (e.g., Pseudomonas) and enrich for NTM. Because NTM grow much slower than other bacteria, laboratory cultivation of NTM from diseased fish generally requires weeks and months (Astrofsky 2000; Herbst 2001; Sakai 2005; Yanong 2003). Lab workers must kill faster-growing bacteria that often contaminate these tissue samples; otherwise these bacteria will grow over the entire culture dish making NTM detection impossible. Lab workers do this by briefly treating (i.e., “decontaminating”) the fish tissue sample with a potent chemical cocktail of sodium hydroxide, the dye malachite green, and a mild detergent before plating the sample onto culture dishes. Even then the culture dish itself usually contains antibiotics to further kill contaminating bacteria. Many NTM are inevitably killed. However, the NTM that manage to survive can now multiply freely on the culture dish without being overgrown by ordinary bacteria.
Water Treatment
Water treatment, like decontamination during laboratory cultivation, selects for NTM and often increases their numbers. This “NTM enrichment” is a common occurrence in drinking water systems (LeChevallier 2004). For example, chlorine/chloramine treatment at one water treatment plant reduced the number of NTM in raw water from 55 per ml to 0.04 per ml. However, downstream in the distribution network, the NTM population had dramatically increased to 700 per ml (Falkinham 2001). Water treatment kills bacteria, including NTM. However, the surviving NTM prosper. They form long-term biofilms in the water distribution pipes and constantly shed small quantities of NTM into drinking water.
One investigation (Angenent 2005) of a hospital’s therapy pool documents how incredibly enriched NTM can become in a clean, disinfected environment. Water in the therapy pool was filtered with multiple pressurized sand filters followed by UV sterilizing filters, and then dosed with the disinfectant hydrogen peroxide. Despite being maintained and monitored according to public health standards, the pool was causing respiratory infections in pool lifeguards. The investigators were eventually able to prove that NTM (mainly M. avium) in the pool water had caused the respiratory infections. They found that the pool water contained 20,000 NTM per ml, which is grossly higher than the NTM range (0.1 to 500 NTM per ml) found in natural waters (Dailloux 1999). The pool water had a low total bacteria count (400,000 per ml), suggesting NTM enrichment in an environment inhibitory to ordinary bacteria.
Interestingly, the investigators (Angenent et al.2005) found only minute traces of NTM in the pool’s sand filters. Because filters collect debris, the filter environment was more nutrient-rich and “bacteria friendly” than the pool water. It supported a more normal bacteria population. The investigators identified the filter bacteria as mostly Sphingomonadaceae, g-Proteobacteria, and b-Proteobacteria. Apparently filter conditions were favorable for normal bacteria growth. The filter bacteria grew well enough to reduce the NTM population to virtual insignificance.
NTM Environments
NTM survive and thrive in nutrient-poor (i.e., “clean”) environments that starve ordinary bacteria. Steinert et al. (1998) showed this experimentally when they placed E. coli and an NTM (i.e., M. avium) in separate containers of starvation media (no nutrients). After 10 days the M. avium population increased 72-fold while the E. coli population decreased 20-fold. Under nutrient-rich conditions, the results would be quite different; on rich lab media, E. coli has a population doubling time of 20 minutes, while M. avium requires a full 15 hours. This means that after 15 hours on rich growth media, a single M. avium bacterium has divided into two bacteria. Meanwhile, E. coli has divided every 20 minutes (or 45 times) and theoretically increased its population from one bacterium to about 40 trillion bacteria!
NTM enrichment under starvation conditions may explain why laboratory breeding facilities, where fish are maintained in ultra-clean tanks and otherwise receive ideal care, have had numerous and devastating MB outbreaks (Astrofsky 2000; Kent 2004; Sanders 2001). I believe that disinfected, clean tanks eventually become enriched with large numbers of NTM. Unfortunately, researchers and fish veterinarians focus more energy on identifying NTM species in diseased fish than monitoring the NTM concentration (number of NTM per ml of tank water). Since all NTM are potential pathogens, the number of NTM that fish are exposed to is critical.
Disinfection
I am not suggesting that disinfection should never be used, but veterinarians and fish breeders need to understand that routine disinfection selects for NTM. Moreover, one can predict that over time disinfection will select for disinfectant-resistant NTM. For example, a 30-minute treatment with 800 ppm chlorine (1/4 cup bleach/gal water) might eradicate M. marinum, but not M. fortuitum entrenched in a biofilm (Table 2).
Disinfected tanks with clean water are deprived of nutrients and organic matter for normal bacteria growth. They provide a perfect environmental niche for NTM. Bottom line: the cleaner the tank, the more NTM.
MB in Aquariums and Fish Hatcheries
In my opinion, mycobacteriosis in home aquariums like mine is not due to the normal NTM flora within the aquarium suddenly becoming pathogenic and preying on a weak fish. Rather, it is due to the introduction of a chronically infected fish carrying large numbers of NTM. In my case, the new fish showed no symptoms for three to eight months. For many months, then, the infected fish were constantly shedding enough NTM into the water to infect vulnerable tankmates. By the time I realized there was a problem, other fish were infected.
Initial Outbreak
During the initial MB outbreak, I used UV sterilizers and euthanized fish showing distress. After the outbreak I believe that normal competition from ordinary bacteria gradually crowded out the remaining NTM pathogens. My aquariums, which all contain soil, snails, and plants, inevitably provide considerable nutrients and organic debris to support normal bacterial growth. Unlike disinfected, ultra-clean tanks, my tanks do not enrich the NTM population.
UV sterilizing filters alone may be insufficient to rid NTM from a grossly infested tank, but they surely helped in my situation. I believe that the surviving fish owe their lives to UV sterilizing filters. Moreover, UV sterilization is equally effective in killing NTM and ordinary bacteria (LeChevallier 2004), so UV sterilization (unlike many disinfectants) will not enrich the NTM population.
Preventing MB Is a Challenge
Commercial fish breeders justifiably dread MB and use every means possible to prevent the disease from entering their breeding facilities. Unfortunately, many of the best-managed facilities emphasize procedures (disinfection and ultra-clean conditions) that inevitably enrich for NTM. Disinfection kills ordinary bacteria more than NTM, setting up conditions that allow the surviving NTM to grow unchecked. Even if the disinfection kills every last NTM in the tank, NTM from outside sources (tap water, fish, air, etc.) will gradually re-colonize the tank. NTM are everywhere in the environment.
The situation for aquarium hobbyists is not so dire. Most hobbyists like me do not maintain ultra-clean, disinfected tanks that would enrich for NTM and promote MB. I suspect that many fish chronically infected with MB have passed through my tanks during the 50-plus years that I have kept fish. None caused problems—until 2004. I have always removed sickly fish from my tanks; I believe it is a good habit.
There is no cure for MB and none on the horizon. Prevention (careful screening and quarantine of new fish) and good management (good care of fish, prompt removal of sick fish, allowing a normal bacteria flora, and using UV sterilizing filters during MB outbreaks) are effective tools.
Two years after the MB outbreak in my tanks, the 26 surviving rainbowfish are doing great. I am no longer afraid to put my hands in the tank.
Special Thanks
In preparing this article, I would like to acknowledge the invaluable help of the following scientists and fish veterinarians: Roy Yanong (University of Florida); Michael Kent (Oregon State University); Joseph Falkinham III (Virginia Polytechnic Institute and State University); Angelo Colorni (Israel Oceanographic and Limnological Research, Israel); and Ivo Pavlik (Veterinary Research Institute, Czech Republic).
References
Angenent, L. T., et al. 2005. “Molecular identification of potential pathogens in water and
air of a hospital therapy pool.” Proceedings of the National Academy of Sciences of the United States of America 102:4860–4865.
Astrofsky, K. M., et al. 2000. “Diagnosis and management of atypical Mycobacterium spp. infections in established laboratory zebrafish (Brachydanio rerio) facilities.” Comp Med 50:666–672.
Bardouniotis, E., et al. 2003. “Biofilm formation and biocide susceptibility testing of Mycobacterium fortuitum and Mycobacterium marinum.” Current Microbiology 46:28–32.
Beran, V., et al. 2006. Distribution of mycobacteria in clinically healthy ornamental fish and their aquarium environment. Journal of Fish Diseases 29 (7):383–393.
Dailloux, M. et al. 1999. “Water and Nontuberculous Mycobacteria.” Water Research 33:2219–2228.
Davis J. M., Clay, H., Lewis, J. L., Ghori, N., Herbomel, P., and Ramakrishnan, L. 2002. “Real-time visualization of Mycobacterium-macrophage interactions leading to initiation of granuloma formation in zebrafish embryos.” Immunity 17:693–702.
Falkinham, J. O., et al. 2001. “Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other mycobacteria in drinking water distribution systems.” Appl Environ Microbiol 67:1225–1231.
Gauthier, D. T., et al. 2003. “Experimental mycobacteriosis in striped bass Morone saxatilis.” Diseases of Aquatic Organisms 54:105–117.
Herbst, L. H., et al. 2001. “Granulomatous skin lesions in moray eels caused by a novel Mycobacterium species related to Mycobacterium triplex.” Infect Immunity 69:4639–4646.
Kent, M. L., et al. 2004. “Mycobacteriosis in zebrafish (Danio rerio) research facilities.” Comparative Biochemistry and Physiology 138:383–390.
Kirschner, R. A., et al. 1992. “Epidemiology of infection by nontuberculous mycobacteria.” Am Rev Respir Dis. 145:271–275.
LeChevallier, M. W. 2004. “Control, treatment and disinfection of Mycobacterium avium complex in drinking water.” In: Pedley, S., et al (eds). Pathogenic Mycobacteria in Water: A Guide to Public Health Consequences, Monitoring and Management. IWA Publishing on behalf of the World Health Organization, London.
Lescenko, P., et al. 2003. “Mycobacterial infection in aquarium fish.” Veterinarni Medicina 48:71–78.
Poort, M. J., et al. 2006. “Molecular characterization of a Mycobacterium species in non-native poeciliids in Hawaii using DNA sequences.” Journal of Fish Diseases 29:181–185.
Prearo, M., et al. 2004. “Mycobacteriosis: emerging pathologies in aquarium fish.” Veterinary Research Communication 28:315–317.
Pro, S. 2003. Mycobacterium marinum: the fish disease you could catch. Internet website: http://www.reefkeeping.com/issues/2003-07/sp/feature/index.htm.
Rhodes, M. W., et al. 2004. “Isolation and characterization of mycobacteria from striped bass Morone saxatilis from the Chesapeake Bay.” Diseases of Aquatic Organisms 61:41–51.
Sakai M., et al. 2005. “Characterization of a Mycobacterium sp. isolated from guppy Poecilia reticulate, using 16S ribosomal RNA and its internal transcribed spacer sequences.” Bulletin of the European Association of Fish Pathologists 25:64–69.
Sanders, G. E., and Swaim, L. E. 2001. “Atypical piscine mycobacteriosis in Japanese medaka (Orzaias latipes).” Comp Med 51:171–175.
Steinert, M. et al. 1998. “Mycobacterium avium Bacilli grow saprozoically in coculture with Acanthamoeba polyphaga and survive within cyst walls.” Applied and Environmental Microbiology 64:2256–2261.
Talaat, A. M., et al. 1998. “Goldfish, Carassius auratus, a novel animal model for the study of Mycobacterium marinum pathogenesis.” Infection and Immunity 66:2938–2942.

.png?h=595&iar=0&w=2781&hash=5FD5E69473BCC22199FBFA2FB71B6033)